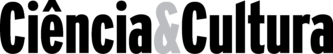Introduction
The “Proalcool” program initiated by the military regime after the two oil crises in the Seventies prompted a search for other renewable resources and their potential uses. Four million cars, vans, and small trucks in Brazil use ethyl alcohol produced by the fermentation and distillation of sugar cane for fuel. This is even mixed (22%) with petrol without damaging engines. On the other side, global society is based on oil, which has been accumulating as subsoil reserves for thousands of years and will be finished soon (estimates 2050). In tropical regions, there is much more sun energy and therefore, chances of replacing petrol by biofuels are better potential alternatives. Progress made in all areas of biomass energy production, has been much greater per unit expenditure than progress achieved in the pursuit of nuclear fusion.[46]
Sugar cane has been grown in Brazil for many decades with low or zero applications of nitrogen fertilizers. There are many areas in the country where sugar cane has been grown for decades, even centuries, and neither cane yields, nor soil N reserves, appear to fall with time, despite this apparent deficit in N supply. These results have led to research concerning the contribution of biological nitrogen fixation (BNF) to the maintenance of cane productivity. Recent results have shown that contributions up to 150 kg N ha-1yr-1 can be obtained from the biological reduction of atmospheric nitrogen. Several nitrogen fixing bacteria colonize the hole plant and some of them live inside the plant which can fix the nitrogen and transfer it direct to the plant tissue. The screening of plant genotype for higher contributions of BNF has been cited to be the key to the replacement of N fertilizers in various important crops like sugar cane, rice, wheat, maize, and others. Also, diesel can be mixed with oil palm (around 20%) without any engine’s modification. African oil palm is the best alternative for the replacement and also is colonized by numerous nitrogen-fixing bacteria. Biofuel are much more compatible with environmental preservation and as a renewable resource must be stimulated by the government.
The Brazilian bio-ethanol program
The elimination of N fertilizers for biofuel crops represents the key to high-energy balances because these fertilizers are produced by the reduction of atmospheric N2 to NH4, using petrol or gas. The Brazilian ethanol program is the best example of biofuel.[16] Sugar cane, grown in Brazil for centuries, never received high N applications and therefore the genotypes grown today obtain significant contributions from biological N2 fixation (BNF). When grown with ample P and K fertilizer and foliar application of molybdenum (500 g ha-1) this crop may obtain more than 50 g N/m2 during three years from BNF which means by extrapolating from the plot size of 2.7 m2, the mean annual contribution to some commercial hybrids of sugar cane CB 45-3 and SP70-1143 a range from 170 to 210 kg N ha-1 (Table 1). These data confirm the differences between plant genotypes.

Table 1. Contributions of biological N2 fixation (BNF) to different sugar cane genotypes, evaluated by N balance and 15N dilution methods during three years
(Source: Urquiaga et al., 1992)
Note: Differences between means significant at P = 0.001. NS, no significant differences between means at P = 0.05
Sugar cane is now planted on 5.0 million ha in Brazil, 9% of the land under agriculture. With mean yields of 64 tons per ha, in addition to sugar, 10-12 billion liters of ethanol are produced per year, equivalent to 200,000 barrels of petrol per day (present situation). Although petrol prices all over the world currently are relatively low, the government of Brazil is convinced of the social and ecological impacts of the biofuel program and plans to support it further. The key to the success of the Brazilian bioethanol program is the high-energy balance obtained in Brazil, as shown in Table 2.
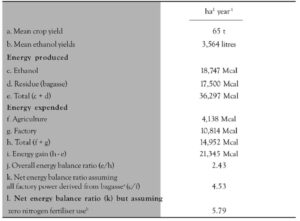
Table 2. Energy balance of ethanol production from sugar cane under Brazilian conditions
(Source: Boddey, 1995)
a Virtually all distilleries derive all heat and electricity from bagasse. Some sell excess bagasse as fuel to other neighboring industries.
b Present mean N fertilizer use 65 kg-1N ha-1 year-1. Estimated energy cost 902 Mcal
Due to the high N contributions the Brazilian sugar cane genotypes obtain from BNF, it is now recommended to the farmers to plant certain plant genotypes, CB 45-3 or SP10-11-43 without any N fertilizer and to use the money otherwise used for N fertilizers for increased phosphate applications, foliar spraying of Molybdenum, the key minor element for BNF, and irrigation. Elimination of leaf burning before harvest also increases the sugar cane yields and reduces the N applications as the leaves can contribute to the maintenance of the N in the system.[40] In addition, it increases soil fertility and reduces irrigation needs. The higher labor need for cutting unburned sugar cane provides more jobs in the interior, and costs are compensated by further increased yields.
The Brazilian Alcohol program has already created more than one million jobs, decreasing the over-populations in large cities. Elimination of cane burning also will further reduce air pollution in addition to the negative greenhouse effect, by removing more CO2 from the atmosphere. The use of biofuels has already reduced the lead content in the atmosphere of large cities by 75% and vehicles running on ethanol have zero lead emissions. Cars running on ethanol also emit 57% less CO, 64% less hydrocarbons and 13% less NOx than cars running on gasoline.[10] The only pollution problem is the smoke and soot produced by burning off the sugar cane trash (senescent leaves) before harvesting. Nowadays, more and more producers are using machinery’s to harvest the sugar cane and around the cities it is already forbidden to burn, reducing further the pollution problems.
Ethanol production increased to 11,900 M liters by 1985 and by 1988, 88% of the new cars being sold were powered by ethanol engines. It reduced drastically in 1995 to 3%. Unfortunately, with the decline in price of petrol, there was no interest of the government to continue this program (Figure 1).
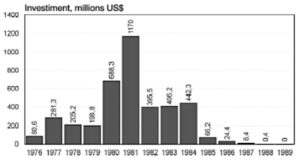
Figure 1. Total Brazilian government investment in the National Alcohol Program (Porálcool) 1976 to 1989
(Source: Boddey 1993)
The key to the success of the Brazilian alcohol program was the continuous selection of plant genotypes with N fertilizer applications much below the plant needs. With this approach, sugar cane genotypes were selected which associate with N2 fixing bacteria using sun energy products of the plant to reduce the atmospheric N2 into NH4. N fertilizers are produced from atmospheric N2 using petrol for the reduction process. High N applications, as recommended by the “Green Revolution” make any bio-energy program senseless because the same amount of energy is used to make the biofuel as is obtained. For this reason, so far, Brazil is the only country in the world where biofuel programs are energetically viable. The overall energy balance of ethanol production in Brazil is 2.5. If bagasse is used to produce all factory power, the energy balance increases to 4.5 and if in addition all N fertilizers are eliminated, it increases to 5.8.[8] With all these practices, Brazil has been using the lowest N applications among all other countries.
Palm oil as an alternative for diesel oil
The African oil palm (Elaeis guineensis) was introduced in Brazil, South of Bahia State, in the XVI Century by the African slaves. Only in the 70’s a stimulus in the production of this oil occurred due to the increase of its price in the world market and Pará State turned to be the highest producer in Brazil. Malaya is the biggest producer in the world contributing with more than 50% of the world production and also is the highest exporter country (64%). Brazil is responsible for only for 0.6% of the world production although palm oil the second one in production (18.49%) and consumption (20.40%) in the world.
The African oil palm seems to be the most interesting crop from the point of view of yield production. It may produce 4.0-8.4 t oil ha-1 yr-1 and it has the highest energy yields (Table 3).
Table 3. The agricultural and energy yields of the most promising crops
(Source: Dobereiner et al., 1981)
a Calculated from present mean yields
b Present means are from extraction of native trees and potential are from cultivated plants
c Calculated on the basis of: ethanol 5,120, methanol 5,034, oil 9,723 kcal/L and wood 3,000 kcal kg and 107 kcal = 1 TOE
Another promised crop is peach palm (Bactris gasipaes) that is also grown in the wet tropics and is already exploited commercially for palm hearts and its high yields of edible fruits. Besides oil, the starch meal can be converted into alcohol. This crop can also produce all over the year as the African oil palm with yield of 4.8 t oil ha-1 yr-1 corresponding to 57 kcal ha-1 yr-1.[43] In contrast, soybean produces only 0.6 t oil ha-1 yr-1 during its crop cycle (120 days). Palm trees, like African oil palm, can maintain a permanent large leaf area and root system and continuously produce, if well managed, for more than 20 years. This crop can also be harvested continuously throughout the year, requires a simple pressing process to produce the final fuel and release much less effluent to dispose of. It can also be grown on extremely poor soils, and it is well adapted to wet tropic climate and represents a desirable tree alternative in forest areas. The main disadvantage is the high costs to establish the crop (1,866.21 US$ ha until the first harvest — Agrianual 97) and the long period of time 4 years it takes until the first harvest.
Attempts have been made to convince the Brazilian government about the advantages of replacement of diesel oil by palm oil, which could create thousands of jobs for the poor population in the North and Northeast of the country. If the African oil palm would be planted in 30% of the area already deforested in the Amazon region, it could produce enough palm oil to replace all diesel oil used in Brazil, which means around 460,000 barrels of diesel per day.[8]
The possibility of running diesel motors on vegetable oil has been known since the work of Rudolf Diesel in 1911. The replacement of diesel oil by palm oil, which is used mainly in trucks and tractors would be of a tremendous beneficial effect to the environment, because oil palms take out more CO2 from the atmosphere than would be returned by trucks, causing a negative greenhouse effect. Diesel oil can be mixed with up to 20% palm oil without any need of change to the motor of trucks and buses. However, a further increase would require considerable modifications to the engine design that are already available and have been sold by a German company Eslsbett Elsbett. Vusof Basiron and Ahmad Hitan estimated the cost of the use of oil palm in a car Mercedes 190 D using an Eslsbett Elsbett motor. A car powered with this engine runs 35,000 km without any technical problem using 6 liters of fuel per 100 km in urban area and à km on the road, with 30% superior performance than the normal diesel. The cost was estimated as 4.80 cents per km for oil palm fuel versus 5.87 cents using diesel.
“The Brazilian ethanol program is the best example of biofuel.”
Like in sugar cane, no high N fertilizer doses were applied on palm trees. The reason might be because commercial plantations of oil palms are mainly carried out in the poorest regions of the country that is, the North East and the Amazon region and because of the very little commercial use of these palms.
Species from the complex Orbignya-Attalea-Maximiliana are observed naturally in 16 million of hectares in Brazil and is normally called Babassu, which is actually the palm, which also has a high extractive potential in the continent. Under natural conditions the productivity reaches around 15 liters of oil ha-1 and has a potential of up to 1 ton of oil per hectare after domestication. Babassu is exploited for the oil rich kernels and also the whole nut can be used as an energy source.[27] There exists approximately 3,000 species of palm trees around the world of which about 1,600 occur in the American tropics region.
N2 fixing bacteria colonizing sugar cane and oil palms
Sugar cane
During the 1950’s two species of diazotrophic bacteria were found in high numbers in the rhizosphere of sugar cane. One of them was a new species called Beijerinckia fluminensis.[15] These bacteria however only occur in soil and therefore the N2 fixed by them is only partially available to the plant. In the 1970’s a new genus, Azospirillum was described which also survives in soil and is enriched in the rhizosphere of various Gramineae including maize, rice, forage grasses, sugar cane and palm trees and which contains some specific strains which are able to infect the plant and multiply within plant tissues.[47]
Only at the end of the 80’s, aerial parts of plants, especially sugar cane that has a lot of carbon in the stems, was used for isolation and quantification of diazotrophic bacteria. This new habitat enabled the discovery of new species that colonize the plant interior without exhibiting any symptom of disease. In 1988, a new species of the Acetobacter family was found inside the sugar cane and was called Acetobacter diazotrophicus.[12, 23] Recently, these kinds of organisms that live inside the plant tissue residing latently or actively colonizing locally or systematically and do not show visibly harm the plant and in some case improving plant growth and reduce disease symptoms caused by several plant pathogens, which do not survive in soil and which are transmitted within plant cuttings or seeds.[32, 13, 21, 34, 41, 26] More recently however, two new species of N2 fixing were reclassified as endophytes,[16, 4] such as Herbaspirillum seropedicae [2] and H. rubrisubalbicans [22] and another new species was described colonizing rice, maize and also sugar cane and was named Burkholderia brasilensis.[6]
A. diazotrophicus was first isolated from sugar cane and since then it was only isolated from Pennisetum purpureum, sweet potato and recently from coffee plants.[45, 31] Comparing the survival outside the plant tissue from these endophytic bacteria, A. diazotrophicus was never found in the soil and inoculations of sterile and natural soil failed to isolate the bacteria.[4] It means that this organism needs the sugar cane tissue to survive and to pass to the next crop. In sugar cane, Acetobacter diazotrophicus was found colonizing the roots, stems, leaves, trash [45] and internally was found in the xylem [30] and in the apoplast space in Cuba.[19]
Using a model system to study the transference of the N fixed by this bacterium Cojho et al., (1993)[14] used a mixed culture with a yeast and observed that more than half of the N2 fixed by the bacteria could be liberated to yeast and suggesting that the plant also can obtain this amount of N. Acetobacter diazotrophicus seems well adapted to these sugar cane tissue as it shows the best growth with 10% sucrose and at pH 5.5. In addition, these bacteria do not pose a nitrate reductase, being able to fix N2 in the presence of high levels of NO3.[12] In the presence of 10% sucrose, the NH4 assimilation by these bacteria is only partially reduced.[9, 44] This bacterium has also its nitrogenase activity only partially inhibited by ammonium [50] and in the presence of 10% sucrose, the enzyme continues to fix nitrogen.[44] Also, in the presence of high sucrose, the inhibition by oxygen, which damage the nitrogenase system, is less sensitive, maybe by the osmotic protection as the diffusion in the level of sucrose (10%) is reduced.[44] These characteristics enable the bacteria to fix N2 in complementation to N assimilation by the plant from soil.
“These new findings open perspectives for a replacement of diesel oil as well as gasoline by bio-energy sources which are much more compatible with environmental preservation and are renewable resources.”
In addition, two new species of Herbaspirillum were also found colonizing endophytically sugar cane roots, stems, and leaves.[5] Herbaspirillum seropedicae was originally isolated from rhizosphere soil, washed roots and surface sterilized roots of maize, sorghum, and rice,[2] but not from uncropped soil.[7] H. seropedicae was originally thought to be a new species of Azospirillum by its similar growth characteristics in the semi-solid, N-free media. However, further analysis showed that it was a new genus.[2] Until now, this bacterium has been reported in 13 members of the Gramineae, normally colonizing roots [38] but was also found in the aerial parts of rice and maize as well as stems of sugar cane, but not in leaves.[38] In 1990, Gillis et al. reclassified Pseudomonas rubrisubalbicans, which causes the mottled stripe disease in sensitive sugar cane varieties, as H. rubrisubalbicans. With this new reclassification, another group was identified as “species 3” but includes only non-diazotrophic bacteria and is mainly isolated from clinical material, such as wounds and feces, although a few strains have been isolated from sugar cane, sorghum, and maize.[3] These two species of diazotrophic bacteria have very similar physiological characteristics, and they can differ only in the utilization of meso-erythritol as sole carbon source by H. rubrisubalbicans and N-acetyl glucosamine by H. seropedicae and these characteristics are used to separate them. Also, the optimal growth temperature (30 oC, H. rubrisubalbicans; 34 oC, H. seropedicae) and by the use of oligonucleotide probes.[3] H. rubrisubalbicans is less common, and could be isolated mainly from sugar cane and in less frequency from sorghum,[24, 25] rice, palm trees [4] and Miscanthus.[33]
Microscopy studies were performed to compare mottled stripe-disease susceptible variety of sugar cane (cv. B-4362 – Barbados) and the resistant one (cv. SP70-1143 – Brazil) and the difference was great. In the susceptible plant, the xylem vessel was completely blocked, intercellular spaces and substomatal cavities by the growth of H. rubrisubalbicans. In the resistant variety SP70-1143, bacteria were restricted to microcolonies encapsulated within polymeric material.[39] James et al., (1997)[29] inoculated this two species of bacteria in sorghum leaves and observed that both posses the same behavior, forming microcolonies in sorghum leaves. A complete review of these two endophytic bacteria was done by James and Olivares, (1997).[29]
Recently, another new endophytic N2-fixing bacterium, Burkholderia brasilensis that grows best at pH 4.5-5.5 was described by Baldani et al., (1996b).[3] This species was also isolated from sweet potato, rice, and cassava. Also, a few strains of another species of Burkholderia were isolated from sugar cane plants collected from Pernambuco. These strains are very close related to B. brasilensis and any the physiological testes could differentiate them until now. The only way is to use the probes produced for each type strain using 23S rRNA variable sequence. This result suggests that these strains belong to two different species (Kirchhof et al., 1997).[33]
The endophytic occurrence of these diazotrophs may now explain the high contributions sugar cane can obtain from BNF observed by Lima et al., (1987),[35] Urquiaga et al., (1992),[51] and Yoneama et al., (1997).[52] The amount of N obtained from BNF and the difference between cane genotypes is shown in Table 1.
Palm trees
Until now, only two reports showed the presence of diazotrophic bacteria belonging to the Azospirillum amazonense species were found colonizing roots of palm trees.[36, 37] Recent, attempts were carried out to isolate similar endophytic diazotrophs from palm trees at various sites in Brazil, including Amazon and South Bahia (Table 4). Numbers of diazotrophic bacteria were higher in roots as compared to the other part of the plants. Also, the endosperm of the seed is colonized by these microorganisms. Oil palm (Elaeis guineensis – Dendê) and peach palm (Bactris gasipaes – Pupunha) are colonized by Azospirillum brasilense, A. amazonense, A. lipoferum, Herbaspirillum seropedicae occurred in oil palm while Azospirillum brasilense, A. amazonense, A. lipoferum and Beijerinckia spp. were found colonizing peach palm. Also, other as-yet-unidentified N2-fixing bacteria were present in these two palm trees. These unidentified bacteria are present in the roots, stems, and leaves and in the endosperm of the fruit. Preliminary results suggest that a new Herbaspirillum species is present in roots, stems, and leaves of these palm trees.[20] In the literature, there is only one report showing occurrence of Azospirillum spp. in oil palms grown in Malaya.[48]
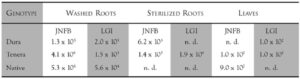
Table 4. Occurrence of diazotrophic bacteria in tree different genotypes of oil palm collected in the south region of Bahia state (numbers of cells per gram fresh weight)
(Source: Carvalho and Dobereiner, in preparation)
JNFB semi-specific media used for isolation of Herbaspirillum species.
LGI semi-specific media used for isolation of A. amazonense
Carvalho, (1997) [11] used a mixture of strains including H. seropedicae (Z67), Burkholderia brasilensis (M130) and A. lipoferum (Sp260) in an inoculation experiment with African oil palm and peach palm plants. The author also used a mixture of 3 isolates from African oil palm, including 2 strains of Herbaspirillum (8A and 7C) and one strain of A. brasilense (23B). These experiments also included a treatment using mycorrhizae fungi Glomus clarum alone or in a mixture with the diazotrophic bacteria. These plants were dependent on N fertilization during the first 6 months, and the AM fungi increased N assimilation by the peach palm and the African oil palm. The inoculation with diazotrophic bacteria alone or with AM fungi showed a better effect than the uninoculated control without nitrogen, but it was lower than the N fertilization for all parameters analyzed.
The author also applied the same treatments to oil palm plants replacing one treatment by a mixture of 3 species of arbuscular mycorrhizal fungi and a mixture of strains: A. lipoferum Sp260 and Br 17; H. seropedicae Z67 and B. brasilensis M130 and the isolates from palm trees. An increase in stem height, stem diameter, height of the first leaf, leaf area and weight of the dry shoot and total N in roots was observed with inoculation of a mixture of all bacterial strains. It suggests that a better combination of diazotrophic bacteria must be tested.
Perspectives for the future
All these new findings open perspectives for a replacement of diesel oil as well as gasoline by bio-energy sources, which are much, more compatible with environmental preservation and are renewable resources. The substitution of these derivatives of petroleum is necessary to overcome these problems and the solution is renewable energy sources, which are clean and originate from biomass. This is only possible in countries that possess sufficient reserves of land for the expansion of crop production along with a suitably warm climate and an abundance of rainfall as observed in Brazil and Nigeria.
“The Brazilian government must stimulate the use of these natural energy sources and also sell this technology or the product (ethanol) for the others, especially to cities where the air pollution is too high.”
In the Conference held in Brazil in 1992 (ECO-92), the developed countries made several promises to reduce the global effects of the use of fossil fuels, but this has not been done. Of course, it is not easy to change the society, as the cost of petrol does not provide the necessary motivation. Also, the countries which are localized in temperate region have their lands are fully occupied. The Brazilian government must stimulate the use of these natural energy sources and also sell this technology or the product (ethanol) for the others, especially to cities where the air pollution is too high, such as Mexico City. Of course, the government must invest money to maintain the progress and reduce the global alterations in America. The use of oil palm oil as a fuel is the best option to reduce the use of imported oil, as it is the requirement for diesel oil that is mainly responsible for the high demand for imported crude oil. Palm oil would be an ecological solution to replace diesel oil imported to the Amazon region.
In any case, an energy balance must be positive, otherwise it is not viable. Biological nitrogen fixation can reduce the use of N fertilizer, which is the most expensive and also needs fossil energy for its production. In sugar cane, even not replacing nitrogen fertilizer by BNF, the cost of production in Brazil are already the lowest in the world. Here, the N fertilization adopted by the farmers averages 60 kg N ha and the productivity in the State of São Paulo (where 60% of Brazilian sugar cane is grown) is around 80 ton fresh stems per ha. In the United States, Australia, Mexico, India (maybe others) the N fertilization is around 200-300 kg ha. In Australia, using 200 kg N/ha and irrigation in much of the area, the productivity is only slightly above that of São Paulo 84 ton ha. In these countries, the energy balance is much less favorable, even negative in some cases, and ethanol production from this crop is not viable.[42]
In Brazil alcohol has been used as a fuel to replace gasoline in cars and light vehicles for almost 20 years and the air pollution of São Paulo city was considerably reduced.[10] Another important reason that we can not forget is that the oil from the African oil palm can be produced in the northern region were the cost of transportation of fuels is high. The PROALCOOL program together with the “DENDIESEL” program needs political support.